The moles grams and molecules worksheet introduces students to the fundamental concepts of moles, grams, and molecules, equipping them with the knowledge and skills to perform essential conversions and solve problems in chemistry and related fields. This worksheet provides a comprehensive overview of these concepts, including their definitions, relationships, and practical applications.
Through engaging explanations, step-by-step calculations, and real-world examples, this worksheet empowers students to confidently navigate the complexities of mole-gram conversions, unravel the significance of Avogadro’s number, and appreciate the versatility of moles, grams, and molecules in scientific research and technological advancements.
Definition of Moles, Grams, and Molecules
The mole, gram, and molecule are three fundamental units used in chemistry to measure the quantity of substances.
A moleis the standard unit of measurement for the amount of a substance. It is defined as the amount of substance that contains exactly 6.022 × 10 23elementary entities. These elementary entities can be atoms, molecules, ions, or electrons.
A gramis a unit of mass equal to one thousandth of a kilogram (1 g = 10 -3kg).
A moleculeis the smallest unit of a compound that retains the chemical properties of that compound. It consists of two or more atoms held together by chemical bonds.
Relationship between Moles, Grams, and Molecules
The mole, gram, and molecule are related through Avogadro’s number, which is 6.022 × 10 23. This means that one mole of any substance contains 6.022 × 10 23elementary entities (atoms, molecules, ions, or electrons).
For example, one mole of carbon contains 6.022 × 10 23carbon atoms. One mole of water (H 2O) contains 6.022 × 10 23water molecules.
Calculations Using Moles, Grams, and Molecules
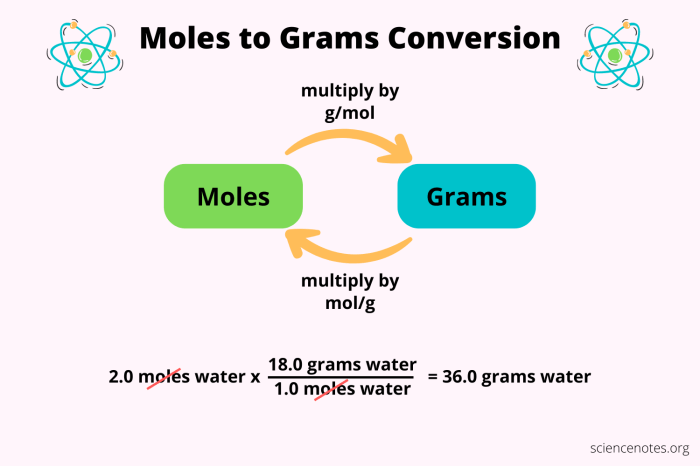
Converting between Moles and Grams, Moles grams and molecules worksheet
To convert between moles and grams, we use the molar mass of the substance. Molar mass is the mass of one mole of a substance and is expressed in grams per mole (g/mol).
The formula for converting moles to grams is:
Grams = Moles × Molar mass
For example, to convert 2 moles of carbon to grams, we would use the molar mass of carbon, which is 12.01 g/mol:
Grams = 2 moles × 12.01 g/mol = 24.02 g
Converting between Grams and Molecules
To convert between grams and molecules, we use Avogadro’s number. The formula for converting grams to molecules is:
Molecules = Grams × (6.022 × 1023molecules/mole)
For example, to convert 24.02 g of carbon to molecules, we would use Avogadro’s number:
Molecules = 24.02 g × (6.022 × 1023molecules/mole) = 1.204 × 10 24molecules
Avogadro’s Number and Molar Mass

Avogadro’s Number
Avogadro’s number is a fundamental constant in chemistry. It is defined as the number of elementary entities (atoms, molecules, ions, or electrons) contained in one mole of a substance.
The value of Avogadro’s number is 6.022 × 10 23mol -1. This means that one mole of any substance contains 6.022 × 10 23elementary entities.
Molar Mass
Molar mass is the mass of one mole of a substance. It is expressed in grams per mole (g/mol).
The molar mass of a substance is equal to the sum of the atomic masses of all the atoms in the molecule.
For example, the molar mass of water (H 2O) is 18.015 g/mol. This is because the atomic mass of hydrogen is 1.008 g/mol and the atomic mass of oxygen is 16.000 g/mol.
Applications of Moles, Grams, and Molecules: Moles Grams And Molecules Worksheet
Chemistry
Moles, grams, and molecules are used in a wide variety of chemical calculations. For example, they are used to:
- Calculate the amount of reactants and products in a chemical reaction
- Determine the concentration of a solution
- Calculate the mass of a substance
- Determine the empirical and molecular formulas of a compound
Biology
Moles, grams, and molecules are also used in biology. For example, they are used to:
- Calculate the amount of DNA or RNA in a cell
- Determine the concentration of a protein solution
- Calculate the mass of a virus
- Determine the molecular weight of a protein
Common Errors and Misconceptions
Mistaking Moles for Molecules
A common error is to mistake moles for molecules. Moles are a measure of the amount of a substance, while molecules are the smallest unit of a compound that retains the chemical properties of that compound.
For example, one mole of water contains 6.022 × 10 23water molecules.
Using the Wrong Molar Mass
Another common error is to use the wrong molar mass when converting between moles and grams. It is important to use the molar mass of the specific substance you are working with.
For example, the molar mass of carbon is 12.01 g/mol, while the molar mass of carbon dioxide (CO 2) is 44.01 g/mol.
Common Queries
What is the relationship between moles, grams, and molecules?
One mole of a substance contains Avogadro’s number of molecules, which is approximately 6.022 x 10^23 molecules. The molar mass of a substance is the mass of one mole of that substance in grams.
How do I convert between moles and grams?
To convert moles to grams, multiply the number of moles by the molar mass of the substance. To convert grams to moles, divide the mass in grams by the molar mass of the substance.
What is the significance of Avogadro’s number?
Avogadro’s number is a fundamental constant that relates the macroscopic and microscopic scales. It allows us to convert between the number of molecules and the mass of a substance.